The 2025 Nobel Prize in Chemistry goes to...
We're switching things up this week with our first-ever guest post! Today's piece comes from the amazing Dr. Aarati, Green Juice's resident illustrator and tricky-science-stuff explainer.
Why the switcheroo? Two great reasons:
- Jon's birthday is on Friday, dangit, and he deserves a week off.
- Dr. Aarati's birthday is on Saturday, dangit, and Jon has repeatedly ignored her polite requests to do a post on this subject.
(Pssst! Wanna make our birthdays extra special? Subscribe to Green Juice, leave a five-spot in the tip jar, and share this newsletter with your nerdiest besty.)
Without further ado, here's Dr. Aarati.
The 2025 Nobel Prize in Chemistry goes to...
This year, the Nobel Prize in Chemistry was awarded to scientists Omar M. Yaghi, Susumu Kitagawa, and Richard Robson for their contributions to the development of metal-organic frameworks (MOFs).
What, pray tell, is a metal-organic framework? And why does it deserve our most prestigious science prize?
You'll find the answer is very... absorbing.
MOFs are a synthetic super sponge
Ever seen a sponge under the microscope? It looks like a really dense thicket. You can see how stuff gets easily trapped in there.
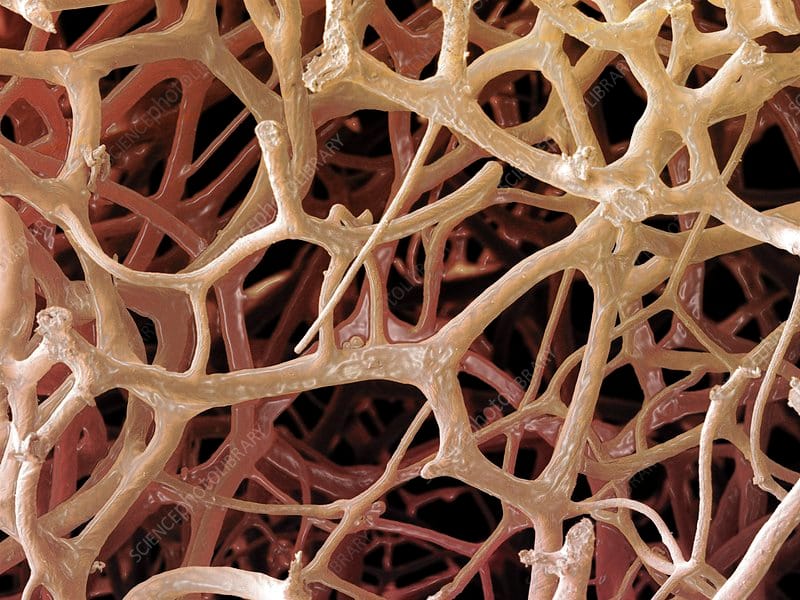
Notice how the gaps in the sponge—the pores—are irregularly shaped. That's nature, for you.
But what if we could design a sponge with a highly precise pore structure? A structure that, due to its shape, only allows specific molecules to get through the thicket... and then traps those molecules inside?
In a nutshell, that's the idea behind the metal-organic framework. Scientists have discovered how to combine tiny bits of metal (ions or clusters) with equally tiny organic “linkers” (molecules) to form a crystalline structure—meaning a shape that repeats identically over and over again.
Picture a spongelike structure made of Tinkertoys. Except, y'know, smaller. A lot smaller. MOFs operate at the nanoscale, meaning MOF pores can be up to 100,000 times smaller than the pores of a sponge.
These intricately constructed frameworks result in a surprisingly massive surface area. Today, we have MOFs with a surface area of up to 7,000 m²/g—about the same area as a football field—encapsulated within the size of a thumbtack.
Now you're starting to see why this incredible work deserves the Nobel Prize!
The simplest version of an MOF might look like this:
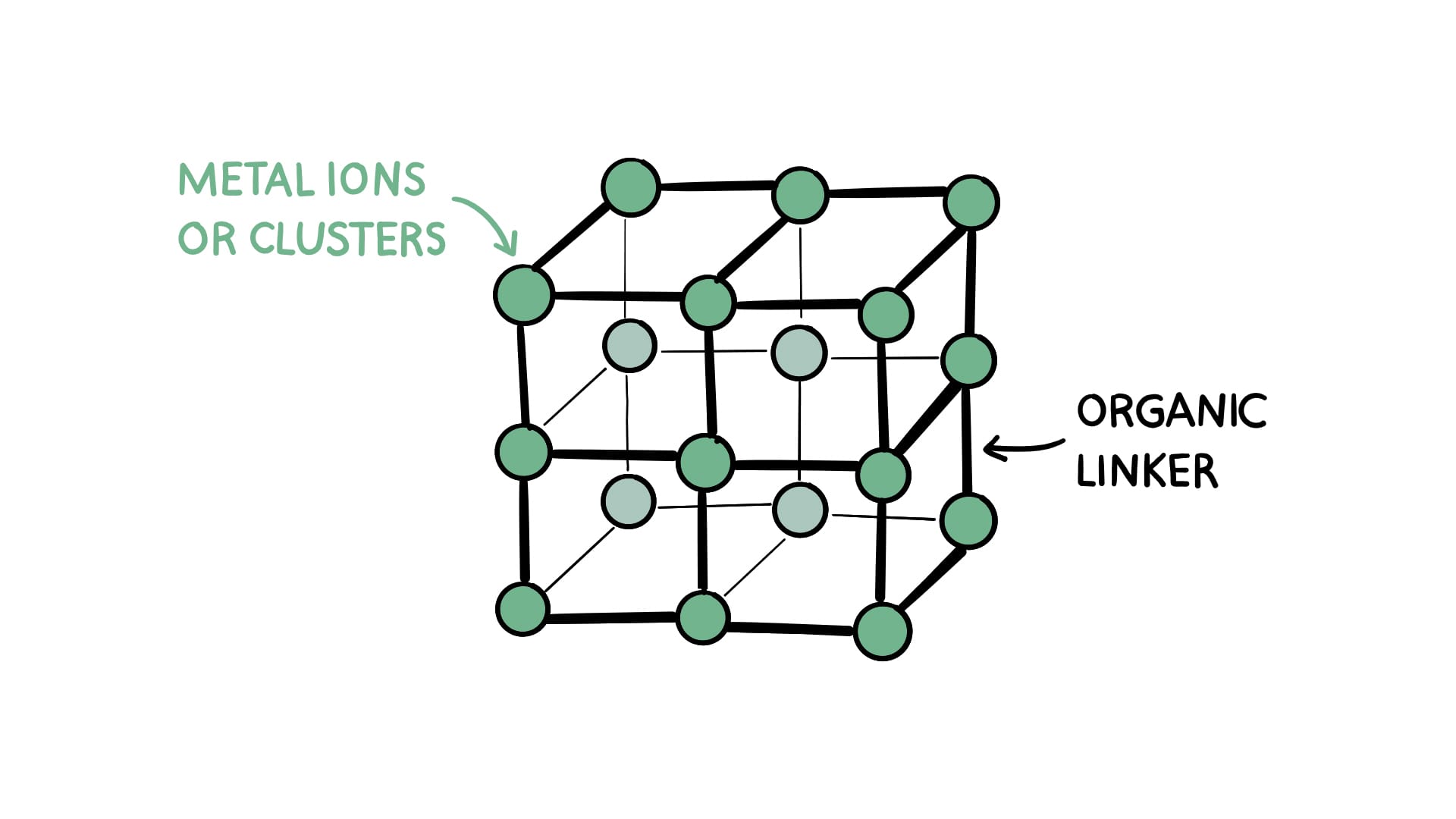
By substituting in different kinds of metals and organic linkers, we can alter the shape of the structure. You end up with MOFs that look more like these:
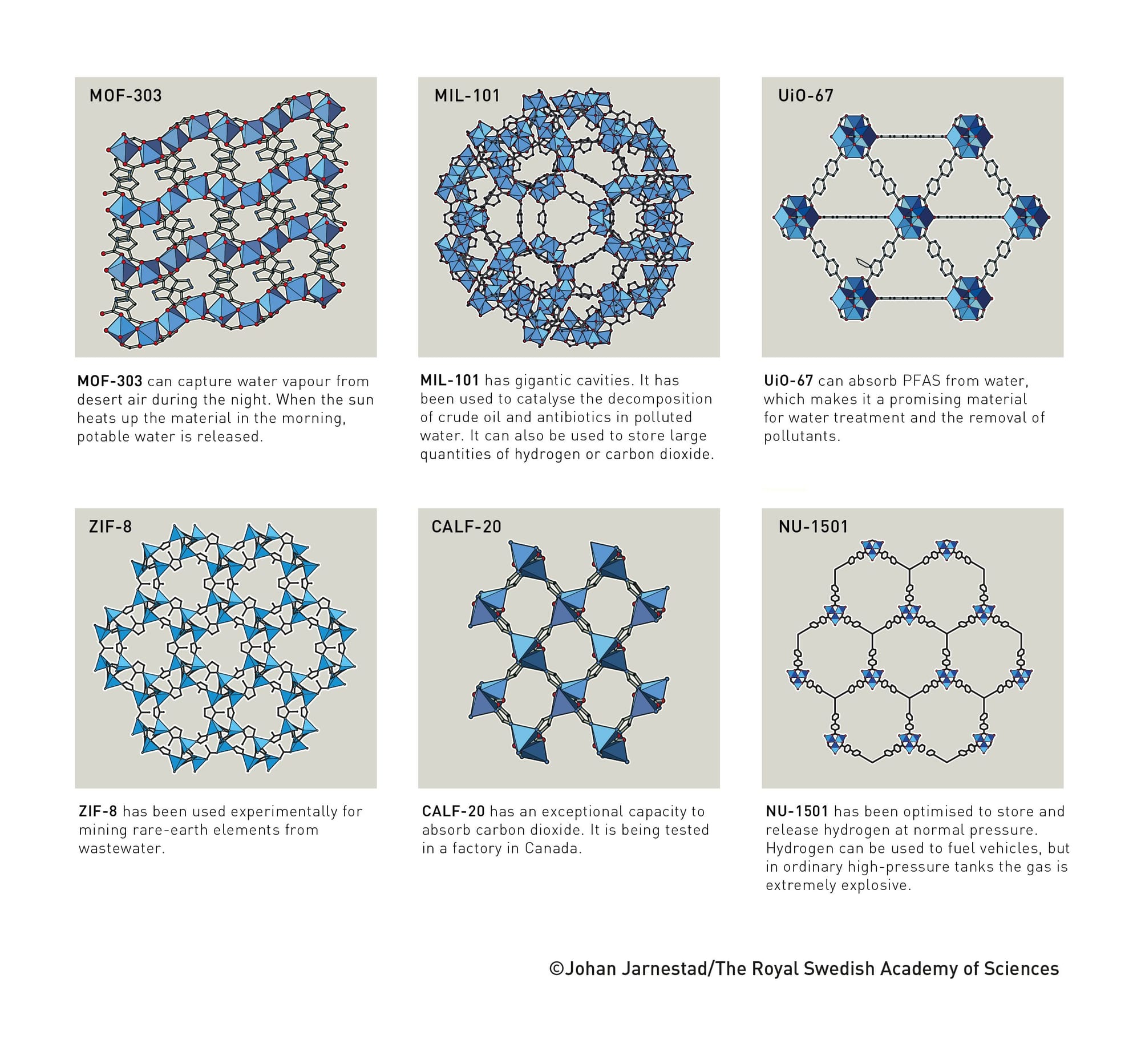
These aren't just pretty Spirograph patterns: they're molecule traps.
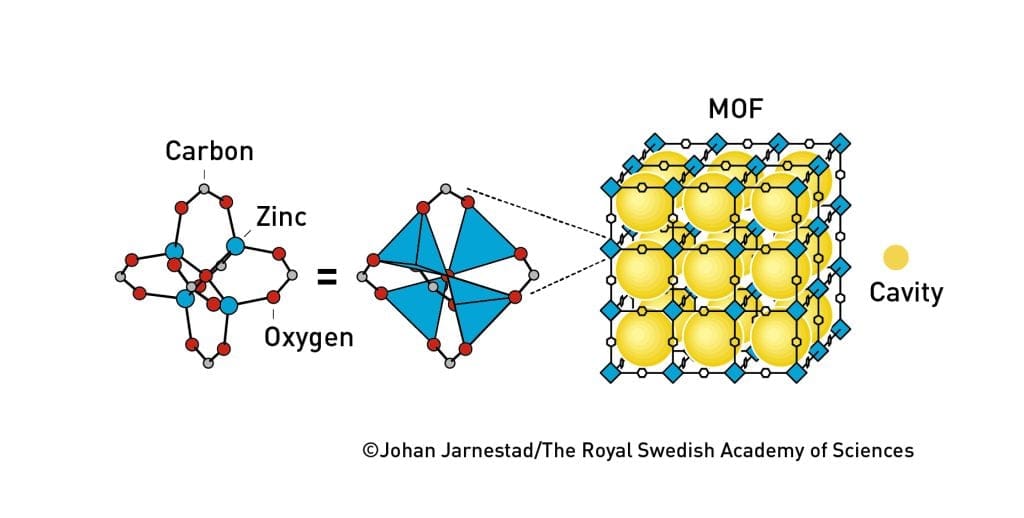
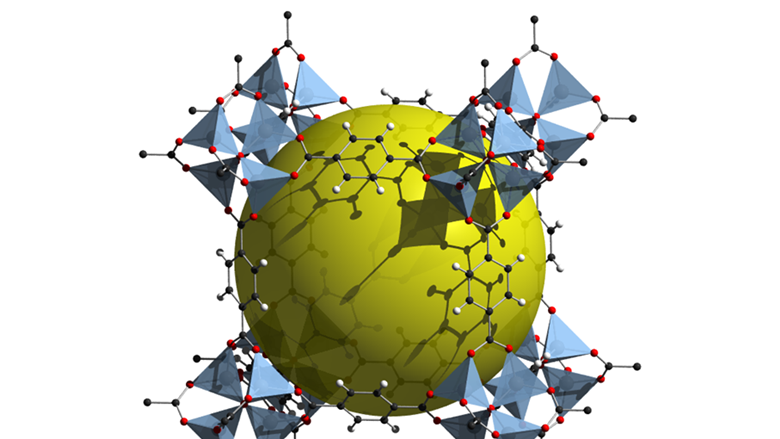
Of course, all this begs the question. Why do we want to trap molecules?
We need to clean up the Earth
Humans are messy... and I'm not just talking about Love Island, people.
We’ve polluted the air with greenhouse gases from agriculture and burning fossil fuels. Carbon dioxide, methane, nitrous oxides, sulfur oxides, and volatile organic compounds (VOCs) are trapping the sun's heat in our atmosphere, preventing it from returning to space. We’ve polluted our oceans and soils with heavy metals from mining, battery waste, and plumbing runoff, industrial chemicals from textile and the pharmaceutical industries, per- and polyfluoroalkyl substances (PFAS) from a number of consumer products like nonstick pans and waterproof coatings, pesticides and herbicides that accumulate in agricultural runoff, and microplastics from all the single-use plastics we use.
Gross!
Each of these pollutants brings its own unique set of problems. For example, while the concentration of CO2 molecules in the air is much higher than we want it to be, it still registers at "just" 426 parts-per-million. Which means that of all the molecules that make up our air, 999,574 are not CO2.
Imagine having 1,000,000 marbles and trying to remove just the 426 bad ones. Not so easy!

Methane gas, which Green Juice readers know is 80 times worse for climate change than CO2, is even less common at only 1.9 parts-per-million.
Or consider PFAS, a.k.a. “Forever Chemicals”. Unlike other water and soil pollutants that we can degrade by using UV light or hydrogen peroxide, PFAS chemicals have strong chemical bonds that make them resistant to degradation.
So how do we get rid of all our pollution?
That answer might just be MOFs.
By creating specialized, molecule-trapping “sponges”, we can selectively soak up these pollutants.
We're gonna talk about exactly how MOFs are being used to do just this. But first, don't you wanna know how they came up with this stuff??
The birth of the metal-organic framework
It started in the early 1990s, when chemical engineers around the world became obsessed with understanding the structure of a family of sparkly minerals called zeolites. (Editor's note: Jon was also obsessed with collecting sparkly minerals in the early 90s.)
There are several types of zeolites, and they all look pretty different to the naked eye:

However, down at the atomic level, zeolites have a lot in common. They're all made of silicon, aluminum, and oxygen, and they have a very precise framework. The aluminum and silicon (metal atoms) act as joints, while the oxygen (an organic atom) acts as a linker between the joints.
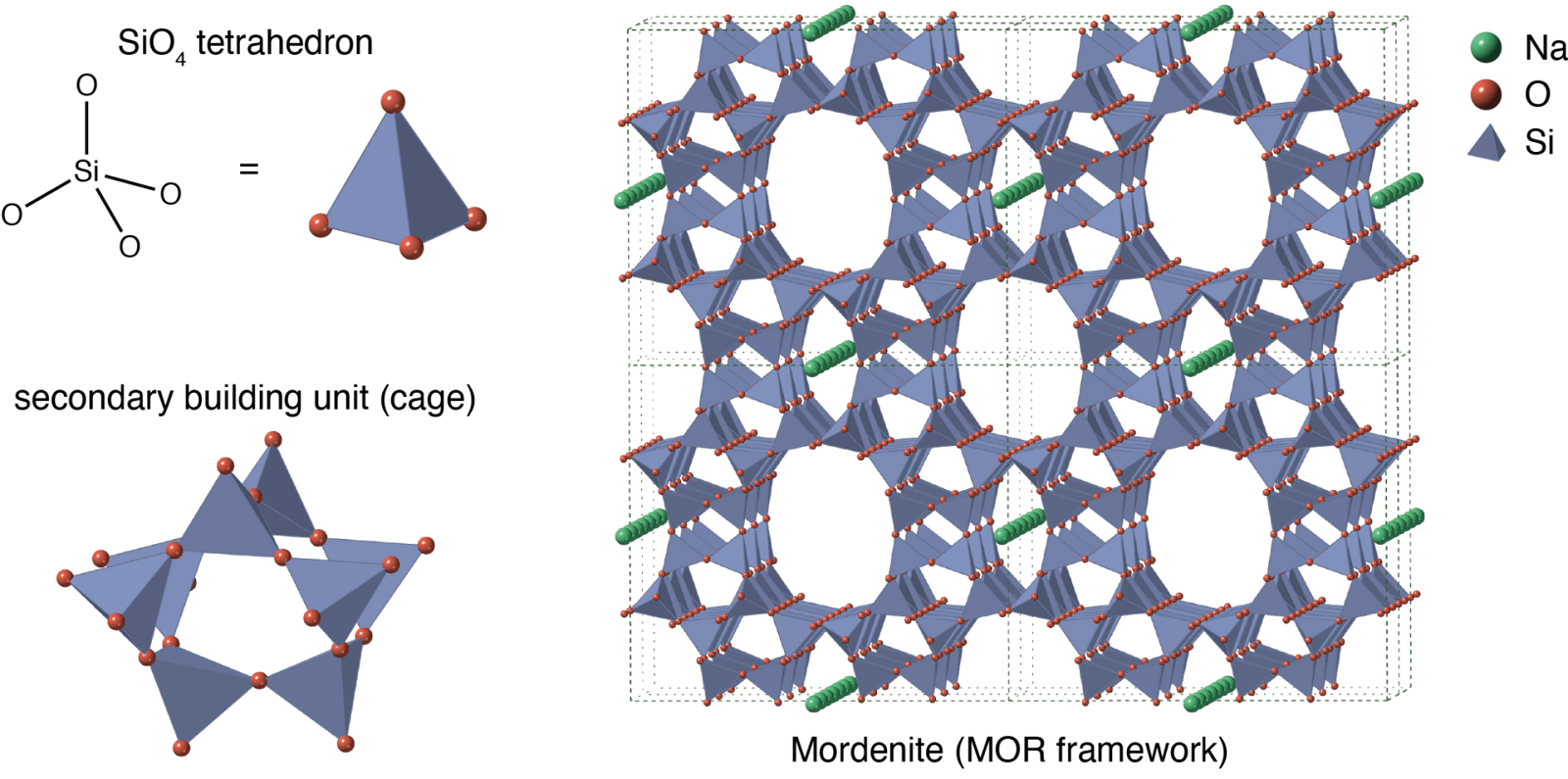
This repeating, crystalline structure gives zeolites an incredibly predictable porosity. How predictable? Well, given a certain amount of zeolite mineral, we can calculate exactly how many holes it has and what its total surface area is.
Zeolites have proven to be fantastic filters and chemical catalysts. They can capture certain heavy metals and ions from water, absorb nitrogen from the air, and catalyze the break down of large hydrocarbon molecules into gasoline and diesel by allowing only specific molecules to enter their pores and then interact with each other.
But they have their limitations, too. The aluminum, silicon, and oxygen atoms that make up the zeolite are great for some things... but not a whole lot else.
So scientists started to wonder: what if we could swap out the silicon and aluminum for different metals? Could the oxygen be exchanged with different organic linkers? Could we create other highly porous materials that still had a predictable, framework-like structure, similar to zeolite, with the ability to do different things chemically?
Easy enough, in theory. In practice, it was anything but. You can’t just throw any random metal ion together with any type of organic linker and call it a day. Early attempts by chemical engineers were unstable. They'd either collapse or form structures that were too small and got clogged, because chemistry has rules and scientists were still figuring those rules out.
In the early 80s and 90s, Dr. Richard Robson at the University of Melbourne, Australia, started laying down some of the foundational chemical and geometric rules for linking metal atoms with organic linkers to form repeating 3D networks. Essentially, Dr. Robson gave us the blueprint for how to build a structure that wouldn’t collapse.
Around the same time, Dr. Susumu Kitagawa was in Japan working on creating metal-organic networks that could hold their shape and absorb and release gases. Dr. Kitagawa showed that these networks could bend and flex while still maintaining their porosity. He gave us the first working model that demonstrated functional structures.
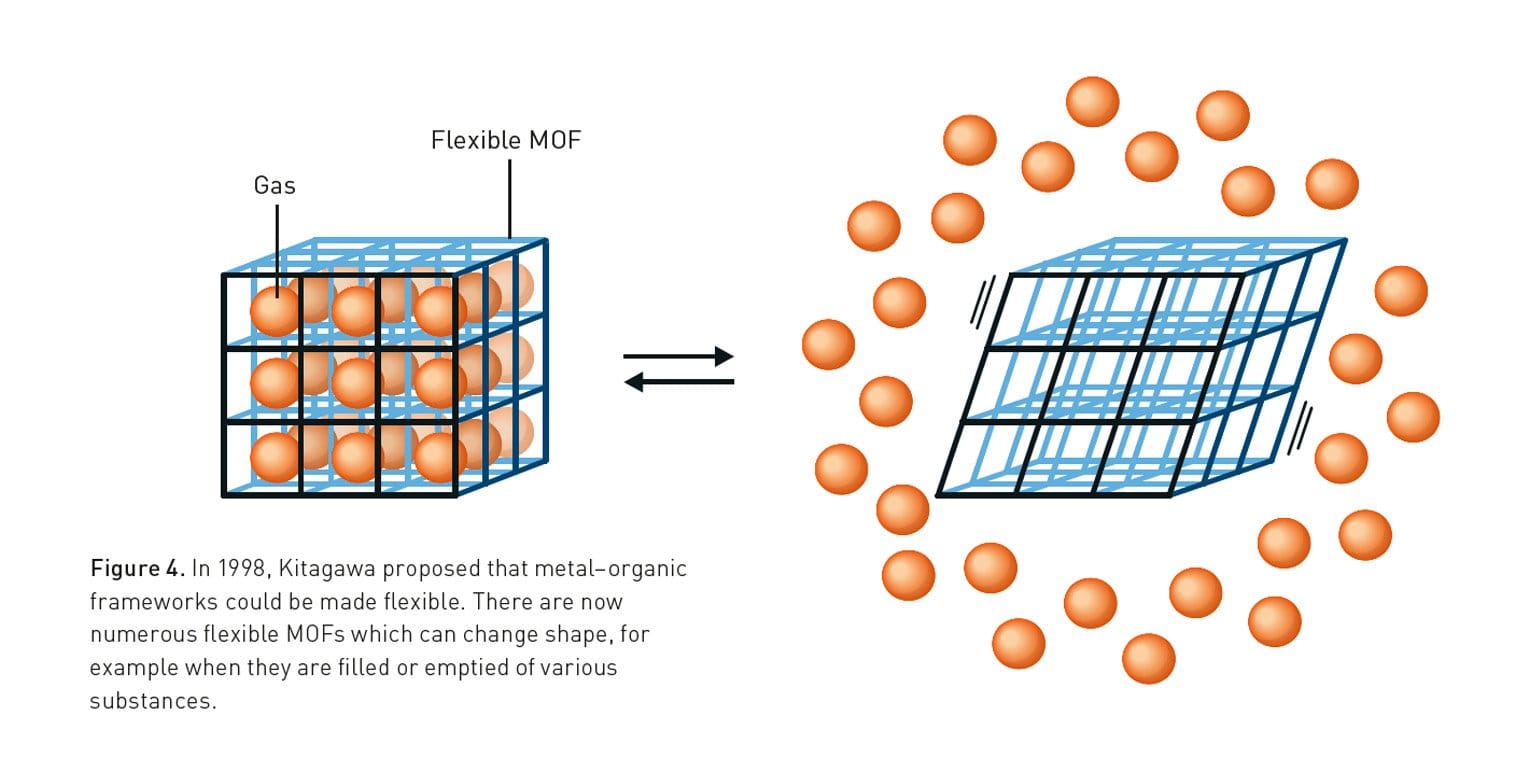
Then, in 1999, Dr. Omar Yaghi and his colleagues coined the term “metal-organic framework” and published the first official one, known as MOF-5. (MOFs 1–4 had all collapsed. Sad.). MOF-5 used zinc ions as the metal and an organic linker called 1-4 benzodicarboxylate (...don’t worry about it). MOF-5 was a huge achievement because it proved MOFs could be made that had large, open pores and wouldn’t collapse. It was able to absorb and store gases, like hydrogen, methane, and carbon dioxide.
Here’s the real kicker. MOF-5 has a surface area of about 2,900 square meters per gram. That's almost 3x more porous than activated carbon or zeolites. That means 3x more gas absorbing power! That’s a lot of area for nifty chemical reactions to take place.
Wow! So how are MOFs being used today?
The work of these three scientists and countless others soon turned MOFs into a major field in materials science. Once we knew the chemical rules and had a working proof-of-concept, scientists got started switching metal ions and linkers like nobody’s business.
Need to capture carbon dioxide from super-hot industrial exhaust streams where normal sorbents don’t work? There’s a MOF for that. Need a sensor that will change color when it detects certain pollutants in the air or water? Yup, there’s a MOF for that.
Here's a non-exhaustive list of what MOFs can be used for:
1. Purification
As mentioned, MOFs have huge potential to help us clean up our environment. We can build MOFs whose pores have a specific affinity for capturing carbon dioxide, methane, PFAS, or any other chemical. And they can work under many different conditions, like extreme temperatures and extreme pressures, where other materials fail.
2. Sensors
We can design “smart” MOFs that change their structure upon capturing certain molecules, allowing them to act as an early alarm system. This structural change can be observed using a variety of methods (X-ray diffraction, luminescence, gas adsorption analysis, etc.). We could use this ability to detect the presence of pollutants, or even to screen for early stages of different diseases, including Alzheimer's and cancer.
3. Gas storage
Because MOFs have such a ginormous surface area, they're basically the best sponges ever. The pores can hold onto huge amounts of methane or hydrogen gas at ambient temperatures without the need for extreme high-pressure compression.
4. Chemical catalysts
The pores in MOFs can be customized to coax certain molecules into their pores, where they'll be forced to interact with each other. Just like zeolites could be used to convert hydrocarbon chains into gasoline, we can now create MOFs that catalyze all sorts of chemical reactions, from synthesis to degradation.
5. Medicine
MOFs can even be used in treatment. For example, you could potentially load up a MOF with chemotherapy and inject it into a cancer patient's body. Upon reaching the tumor, the MOF would release the chemotherapy directly onto the target, minimizing negative side effects.
So there you have it!
Do you know of any other potential use cases for MOFs? I’d love to read them in the comments below.
I hope this article was interesting and, who knows, maybe we’ll do it again sometime. (Editor's note: Yes, please!) But for now, I’m very much looking forward to handing the keyboard back to Jon.
Cheers!
–Dr. Aarati


Member discussion